Abstract
Introduction
Platelets have been implicated in promoting metastasis in a number of cancers. In many solid tumours including colon and ovarian cancer, thrombocytosis has been demonstrated as an adverse factor for disease metastasis and survival. A number of mechanisms have been proposed including platelet "cloaking" of tumour cells to evade recognition by immune cells, production of cytokines which promote tumour growth and the role of platelets in enabling tethering and migration of metastatic cells. In vitro studies have demonstrated the dependence on platelets for successful solid tumour metastasis and implicated the role of P-Selectin in tumour adhesion to platelets. There have been no published studies demonstrating the interaction of platelets with Myeloma cells and their role in enabling Multiple Myeloma (MM) cell metastasis. We sought to determine in vitro whether platelets interacted significantly with MM cell lines and whether they preferentially bind to specific sub-populations of MM cells. We also sought to determine whether this interaction is mediated by P-Selectin and whether "platelet coating" can protect MM cells from Natural Killer (NK)-mediated cell death.
Methods
Platelet rich plasma (PRP) sample was isolated from freshly drawn peripheral blood samples from healthy participants by FICOLL gradient separation. All samples were collected with informed consent and in accordance with the declaration of Helsinki. The MM1S cell line and its derivative HECA-452 MM1S, which is enriched for the sialofucosylated structure sialyl Lewis X (SLex), were used for this study. Cells were co-cultured with PRP at a ratio of 50 platelets/ 1 MM cell, washed and stained with CD41, CD138, and HECA-452. FACS was used to assess degree of platelet/MM cell binding. Platelet/MM cell interaction was further confirmed using Image Stream. In some experiments, a P-Selectin blocking antibody was used to determine the dependency of platelet/MM cell binding on P-Selectin. For functional studies, MM cells were co-cultured with PRP at different platelet/cell ratios (50:1, 100:1 and 500:1) for 24 h and then incubated with either the NK cell line KHYG-1 or freshly isolated autologous NK cells. Cytotoxicity assays were performed after 24 h by FACS using Propidium Iodide (PI) staining. NK cells were discriminated from MM cells by staining them with cell trace violet dye.
Results
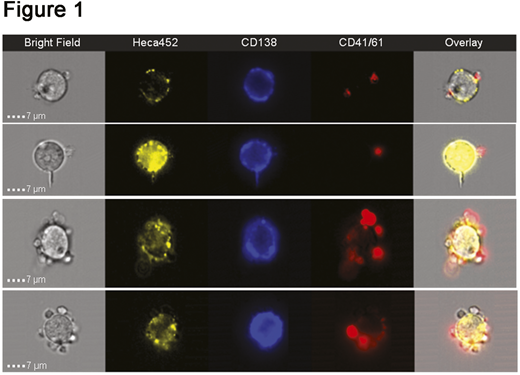
Platelets displayed preferential binding to the HECA-452 MM1S cells compared to parental cells, suggesting a requirement for sialofucosylated structures for efficient binding. The interaction between platelets and HECA-452 MM1S cells occurred as early as 30 min and remained stable at 24 h post incubation. Moreover, platelets bound to the surface of MM cells, as demonstrated by Image Stream (Figure 1). This binding could be significantly blocked by a P-Selectin blocking antibody, indicating a dependence upon P-Selectin (P=0.0043 at 50:1; P=0.0027 at 100:1; P=0.0163 at 500:1 ratio). Importantly, co-incubation of platelets and MM cells at 500:1 ratio significantly reduced KHYG1-mediated cytotoxicity on HECA-452 MM1S cells at 24 h post incubation (P=0.0078 at 0.25:1 and P=0.0072 at 0.5:1 ratio). Platelets could also protect HECA-452 MM1S cells from primary NK-mediated cytotoxicity, although this didn't quite reach statistical significance.
Conclusions
We demonstrate for the first time a physical interaction between MM cells and platelets. Efficient binding requires sialofucosylated structures, as shown by almost exclusive binding of platelets to the HECA-452 MM1S compared to parental cells. Moreover, platelets were able to protect HECA-452 MM1S cells from NK-mediated cell death, suggesting that in vivo these cells may escape the immune system and promote MM spreading and metastasis. Notably, platelet-MM cell binding was reverted by a P-Selectin blocking antibody, suggesting that this interaction can be therapeutically targeted in order to restrict MM metastasis and re-sensitize MM cells to NK cells.
O'Dwyer:Abbvie: Membership on an entity's Board of Directors or advisory committees; Glycomimetics: Research Funding; BMS: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Onkimmune: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.